
Tech Articles
Particle size characterization is one of the key areas involved in quality assurance. The concept of particle size and size characterization acts as a foundation for all the processes involved in the production of a formulation; from manufacturing to quality control operations. Particle size characterization dictates many properties of the finished product. Particle size characterization of samples is important to make a better quality product, improve its appearance, taste, texture and shelf-life. There are many instruments for particle size characterization that are available commercially. Each instrument is based on a different technique and each technique is based on a different principle. Selecting the right particle size characterization technique for the given sample is a challenging decision. The choice of technique is made according to the sample. Sometimes even a combination of techniques is used to obtain accurate results. There are several factors upon which choice of technique depends like size range, sample quantity, cost-effectiveness etc. To ensure appropriate quality standards in the field of particle sizing and particle size characterization, ICH and US-FDA have recently insisted on including Quality-by-design approach in the pharmaceutical industry. Application of QBD approach to particle size characterization techniques ensures a resilient method which gives reproducible results. It aids in reducing result and method variations and promotes productivity and quality.
Particle size and size characterization knowledge act as a prerequisite for all the processes which are involved in the production of a formulation. They influence mechanical strength, electrical and thermal properties of the finished product. Massive production losses can occur if particle sizes of the materials being used in the process are not appropriately monitored. A wide range of materials starting from proteins and polymers, microemulsions, viruses, droplets, pigments up to sand and cement require particle characterization. Particle size characterization is about describing particle sizes in a formulation that helps in understanding, predicting and optimizing pharmacokinetic properties of that formulation. Accurate determination of particle size is necessary for pharmaceutical industry. It is a physical parameter that must be specified, examined and managed right from the starting material to the finished product. Despite the modern instrumentation, there are some challenges faced in the field of particle size characterization - Need for an appropriate method development specific to drug type, form, and delivery. The very minute quantity of sample available, especially, in initial stages of drug development. Inaccuracies in data interpretation due to most of the particles being non-spherical in shape. Problems occurring during analytical measurements like agglomeration or de-stabilization.
The main purpose of particle size characterization in the pharmaceutical industry is to collect quantitative data on mean particle size, particle size distribution, and particle shape. The other purpose is to ensure the quality of the finished drug product. This applies to powders, suspensions, emulsions, and aerosols. Flow and compaction properties of the powders are influenced by particle size and particle shape. Smaller particles have better dissolution profile and spherical particles have better flowability. Aerosols with droplets having an aerodynamic diameter of 2-5 micrometers will penetrate deeper into the lungs. Particle size also greatly affects physicochemical and bio pharmaceutical properties of drug materials and final dosage forms like absorption, dissolution, bio availability, flow properties of the drug. For example, micronization of drugs like chloramphenicol, griseofulvin results in better absorption profile. But reduced particle size in case of lipophilic drugs like aspirin increases their effective surface area, thereby, decreasing their absorption manifolds. Particle size characterization in the powders ensures powder bed with appropriate and uniform particle size. It improves flow properties of the powder particles. Moreover, proper size characterization of drug materials helps in detecting contamination. Particle size characterization techniques can detect any deviations from the anticipated particle size range and hence confirm the presence of an impurity. For these reasons, particle size characterization is an important parameter in quality control of pharmaceuticals and in the improvement of particle properties.
Particle size characterization can be done using various commercially available instruments. Different instruments are based on different techniques, sometimes even a combination of techniques. Generally, the sample to be introduced into the instrument is first dispersed in an appropriate medium. The dispersion can be either wet or dry. Wet dispersion involves changing the solid-air interface into a solid-liquid one, that is, the sample is first dispersed in a liquid medium. Then, agitation and mixing are done to break down the agglomerates. Lastly, the dispersion is stabilized by adding suitable surfactants. On the other hand, dry dispersion involves particles dispersed in dry form. Agglomerates in dry dispersion are broken by sheer and mechanical forces. For efficient separation, air is passed through the sample before its introduction into the instrument. There are many factors which are considered before choosing one of the two dispersions. For example, the solubility of the sample in water, agglomeration tendency, solvent compatibility, hygroscopicity, fragility and toxicity of the material.
Techniques of Particle Size Characterization:
Laser diffraction technique
This technique can be more appropriately called Low Angle Laser Light Scattering (LALLS). This is now a preferred method for particle size characterization and quality control in many industries. According to ISO 13320, the particle size range where this technique is applicable is 0.1-3000 μm. Laser diffraction technique by static light scattering is based upon Mie theory of light scattering which also includes Fraunhofer theory. According to Mie theory, the intensity vs angle relationship obtained from scattering of laser light, is related to sizes of the particles participating in scattering, with other variables kept constant. The variables include the wavelength of the incident laser light and relative refractive index of sample and dispersion medium. The technique is based on the interaction between light and particle surface. Laser diffraction analyses particle sizes by determining angular variation in light intensity as the laser beam passes through a given sample. Bigger particles scatter light at smaller angles and smaller particles at wider angles. Many particles together form a pattern of scattered light which can be converted into a particle size distribution curve. Utilizing Mie theory of light scattering, the particle size is measured as Volume-weighted distribution. This method is based on the fact that diffraction angle is inversely proportional to particle size. The Fraunhofer approximation gives the following information -
Wavelength of light used is much smaller than particle
All particles disperse light with equal magnitude There is
no penetration of light
The optical model employed in Fraunhofer approximation states that particles are assumed –
To be spherical
To be non-transparent and impenetrable
To scatter light equally at wide and narrow angles
To communicate differently with light as compared to medium.
The above-given model restricts the choice of Fraunhofer approximation for particle size characterization. But Mie theory of light scattering fulfills these drawbacks. It states that -
The particle is assumed to be spherical
The particle is present in homogeneous dispersion Refractive
indices of both particle and medium are known.
Such principle instrumentation includes:
Optical bench- It is where laser beam strikes the particles. Laser source commonly employed is a He-Ne gas laser as they are temperature-stable and have a better signal-to-noise ratio. Detectors are also present to analyze the intensity of light scattered by particles. Photosensitive silicon with a series of detectors is generally used. There is always an optimum number of detectors which can be used, like 16-32. Increasing the numbers does not lead to a better resolution.
Sample dispersion units- These units analyze the samples in their dry or wet dispersions. They guarantee that the sample reaches the optical bench at an optimum concentration. Wet dispersion of sample requires a liquid vehicle - aqueous or organic solvent whereas dry dispersions require only dry air as a dispersant.
Instrument software- This consists of data controlling system which converts the pattern of scattered light into a readable particle size distribution curve.
Laser diffraction technique instrumnents have below advantages:
It has broad particle size analysis range.
It offers flexibility in sample presentation.
The whole of the sample can be analyzed and recovery is also possible.
The technique is fast and gives reproducible and reliable results.
There is no need for calibration in this technique, though validation is required.
This technique does a direct measurement of dry powders.
Laser diffraction is flexible in operations.
Laser diffraction technique instrumnents have below disadvantages:
This technique is comparatively low in resolution.
In this technique, particles with different optical properties cannot be analyzed.
This technique is not for strongly absorbing materials.
The validity of results depends upon the validity of data collected.
There is high obscuration due to the high concentration of particles analyzed.
There is over-estimation of volume diameter for nonspherical particles.
Prior knowledge of refractive index is required.
In this technique, the equivalent diameter is calculated which is not directly related to particle volume.
Formulation ingredients may affect the data in this technique.
This method is mainly applied to low concentration samples to minimize multiple random scattering of the laser beam as otherwise, particle size distribution data would be so difficult to interpret. For the particles with a size below one micron, the refractive index of sample material and dispersion fluid are required to make Mie theory algorithmic corrections.
2. Dynamic Light Scattering
Dynamic light scattering (DLS) is also known as Photon Correlation Spectroscopy (PCS) or Quasi-elastic Light Scattering (QELS). It measures particles in the range of 0.3nm-8μm. Apart from measuring particle size, this technique also measures zeta potential and molecular weight. The basic principle involved is that Brownian motion of particles in a suspension causes scattering of the laser beam at different intensities. These fluctuations correspond to velocity of Brownian motion and therefore, particle size can be determined using Stokes-Einstein equation:

Where,
Dh: hydrodynamic diameter;
Dt: translational diffusion coefficient;
KB: Boltzmann’s constant;
T: thermodynamic temperature;
n: dynamic viscosity.
When a monochromatic beam of light strikes a suspension containing the particles, the light gets scattered and spreads out in all directions. The particles in a suspension undergo Brownian motion. This motion causes changes in the distances between the particles and therefore, causes fluctuations in the phase of scattered light. So, the net result comes out to be fluctuating scattered intensity. The larger the particle, the slower the Brownian motion. It is also used for measuring zeta potential of a particle or estimating the molecular weight of organic compounds. The laser light strikes the sample in the cell and the scattered light is captured by detectors, either at 90◦ (a right angle) or 173◦(back angle). The signal can be deciphered by auto correlation function. The particle size is measured in terms of hydrodynamic diameter, which is the diameter of a sphere which has same translational diffusion coefficient as the particle.

Advantages and disadvantages of dynamic light scattering
3. Automated Imaging
It is an automatic image capturing technique in which individual particle pictures are captured from their dispersions and are analyzed for their respective sizes, shapes or other properties. This technique is of 2 types- Static automated imaging- in which the sample is kept stationary, as on a microscopic slide, Dynamic automated imaging- in which the sample is in motion, as in flow through the cell. It is a high-resolution technique and measures particle size in the range of 1μ to millimeters.
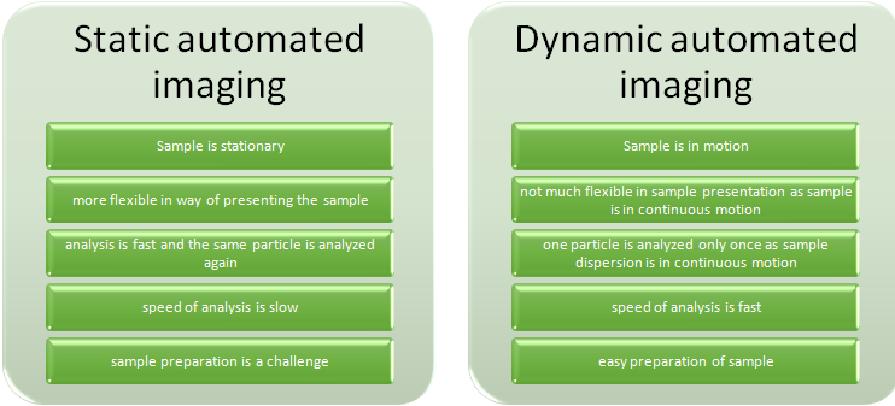
Differences between static and dynamic automated imaging
It employs a digital camera which clicks 2D images of sample particles in the dispersion. Then particle distribution data is generated to report the results. This technique has an upper hand over other techniques as it can measure shape along with particle size. Particle shape measurement is important as it gives idea about whether the particle under consideration is a bigger particle or an agglomerate shape reflects physical properties of a particle so it can be altered if required helps in modifying the particle so as to improve powder flow.
Such principle instrumentation includes:
Sample preparation and presentation- The sample is presented in the form of a dispersion with 2 techniques- static or dynamic. In static imaging, the sample is poured on a microscopic slide, a glass plate or filter membrane and then viewed whereas, in case of dynamic imaging, a flow-through cell apparatus is employed.
Imaging apparatus- a digital CCD camera is employed with desired magnification to capture 2D images of sample particles in the dispersion. Sample illumination is more flexible in case of static imaging as the sample is stationary and a number of techniques can be applied. On the other hand, the sample is essentially illuminated from behind in case of dynamic imaging as the particles are in motion and can only be illuminated from behind.
Data recording and interpretation- Shape and size of each particle are recorded, generally in the form of graphs and curves for easy interpretation.
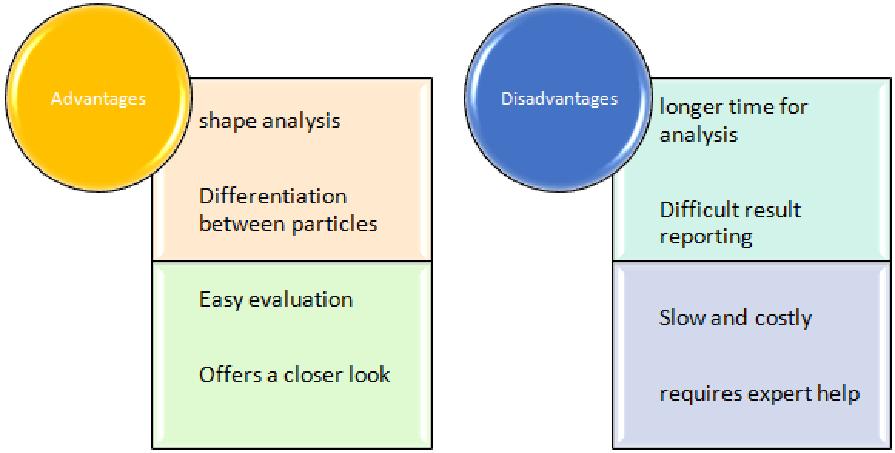
Advantages and disadvantages of Automated imaging
4. Electron Stream Sensing Zone Technique (Coulter Counter Method)
This technique was primarily invented by Wallace H. Coulter of United States Navy, to count blood cells. Its original model measured alterations in electrical conductance as each blood cell dispersed in an electrolyte solution, made way through an orifice. This original blueprint of the technique is known as the ‘Coulter Principle’. This is a high-resolution particle size characterization technique as every single particle passing through the orifice is counted and measured. In this, the particles to be measured for their respective size have to be dispersed in an electrolyte solution. An electrolyte solution is preferred as electrical conductivity is required. Any organic liquid with good electrical conductivity can also be used. The particles are dispersed in this electrolyte solution in low concentration. After that, the particles, essentially one-by-one, make passage through an aperture/ orifice which is located in the wall of an electrical insulator tube called the aperture tube. Electric current is applied across this aperture, thereby, creating an electrical sensing zone. When a particle enters this aperture, it displaces a volume of electrolyte equal to its own volume. This results in the generation of an electrical impulse for a short duration. In this apparatus, two electrodes are employed; one inside the aperture tube and another one outside. This creates a pathway for electrical conductance whenever an external electric field is applied. The alterations in the current are detected, often measured as impedance. From this method, particle volume is obtained, from which spherical diameter can be calculated and hence particle size distribution data can be generated. The particle size range for this technique is 0.2μm to 1600μm. If there are a variety of particles to be characterized, then two apertures instead of a single one are employed.
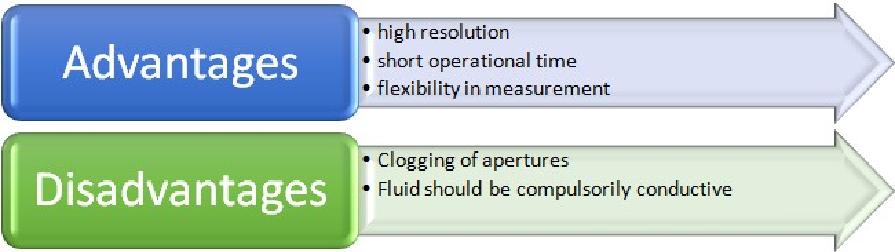
Advantages and disadvantages of Coulter-Counter techniques
5. X-Ray Sedimentation
The X-ray sedimentation technique has garnered a lot of attention and found a bunch of applications since its manifestation in 1967. This technique is primarily grounded on two theories- the sedimentation theory and the X-ray absorption theory. The analytical equipment which works on this principle is called Sedi Graph.
The SediGraph works mainly on two principles. The first principle is the Sedimentation theory which is governed by Stokes’ law. It states that when a particle homogeneously dispersed in a liquid experiences gravitational force which gets equally balanced by buoyant and drags force, then it achieves a velocity called terminal velocity. This velocity depends upon size (diameter) and density of the particle. So alternatively, it can be quoted that the terminal (settling) velocity is directly proportional to a particle size of the drug. The equation for Stokes’ law can be written as-
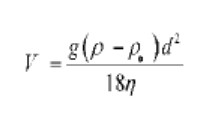
Where,
V: terminal velocity of particle;
G: acceleration due to gravity;
ρ: density of medium ;
ρ.: density of the particle;
d: diameter of the particle;
6 : viscosity of medium
So, if we know the time taken by the particle to travel a known distance, then its velocity can be easily determined by dividing distance traveled by time. Finally, when velocity is known, the diameter of the particle can be easily determined.
The other theory of X-ray absorption aids in the determination of particle concentration. The X-ray absorption technique is based on Beer-Lambert law which states that when an X-ray beam passes through a given sample, its intensity reduces and this reduction in intensity is directly dependent on the concentration of the sample. Alternatively, X-ray intensity decrement is directly proportional to the mass concentration of the sample. Beer-Lambert law has following expression-
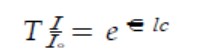
Where,
T: Transmittance;
I: Final intensity of x-radiation;
I.: Initial intensity;
ε: Molar extinction coefficient;
l: thickness of the medium;
C: concentration of the sample in solution.
The value of the intensity of x-radiation is maximum with a clear solution and minimum with homogeneously dispersed particles. So during the process of sedimentation, x-ray intensity rises from minimum to maximum.
In the SediGraph method, a homogeneous dispersion is prepared of particles under consideration in a suitable liquid medium. Agitation is done to achieve this. Then agitation is halted and all the particles are given time to settle down under gravity. The bigger particles settle first and smaller in the last. Each particle settles down with its own terminal velocity which is determined by taking the ratio of distance traveled by particle below a measurement zone to the time taken to cover this distance. When velocity is calculated, the diameter of the particle can be easily calculated from the equation of Stokes’ law. Simultaneously, as the particles settle down in the medium, the intensity of x-ray beam passing through medium also increases. This is because there is no hindrance in the path as the solution above is clear. This increment in intensity relationship with the concentration of the sample can be determined from Beer-Lambert law.
X-Ray Sedimentation instruments have below advantages:
It gives reproducible results.
It allows usage of different liquid mediums.
A small quantity of sample is required.
Size determination is direct in this technique.
X-Ray Sedimentation instruments have below disadvantages:
There are issues of sedimentation instability in this technique.
Frequent deviation from Stokes’ law is also a challenge.
Selecting an appropriate particle size characterization technique out of all the available options is a challenging task. There are several techniques to choose from and also, significant attention to be paid to technique- to- technique variations. The notion that one particular technique is suitable for all the tasks is not a true one. The variations may come because of differences in dynamic range, algorithmic or mechanical improvements in the instruments.
Switching from older techniques like sieving to advanced ones like laser diffraction can also be the cause of variations. Data generated from sieving is comparatively smaller than that obtained by laser diffraction. Microscopic and image analysis techniques are best suited when particle shape is the main area of interest. In case of dispersions and suspensions, when particle size, as well as zeta potential, are the areas of interest, then Dynamic Light Scattering technique is the method of choice.
Reference List:
Jillavenkatesa A, Dapkunas SJ, Lum L-SH. practice guide Particle size caracterization, Nist. Special pu. 2001; doi:Particle size characterization.
Informatics H. Course # Course #.2011.
Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL. Particle size analysis in pharmaceutics: Principles, methods and applications, Pharm. Res. 2007.
Scientific H. a Guidebook To Particle Size Analysis, Distribution. 2010.
Brummer H. Particle characterisation in excipients, drug products and drug substances. Pharma Times.2010.
Choosing between wet and dry dispersion.
Rawle A. Basic principles of particle size analysis. 1993.
Europe CPSI. Comparison of Particle Sizing Methods.
David BF, Weiner. A Guide to Determination of Particle Size – Making an Effective and Reliable Measurement.
Spectroscopy PC. Scattering QL, Motion B. Dynamic Light Scattering.(DLS) DLS Measurement – Scattered Light Intensity Fluctuation, Malvern Guid. (2011).
Saleh OA, Sohn. LL, Introduction I, Coulter counter, Rev. Sci Instrum.2001.
Plain MP. Advantages and Disadvantages of Different Laxatives. (n.d.)2-3.
Law B. The Sedi Graph Method of Particle Sizing. 1877.
Ceramic Industry, Sizing Particles with X-Ray Sedimentation, 2004.
Laidlaw I, Steinmetz M. Introduction to Differential Sedimentation, Anal. Ultracentrifugation Tech Methods.2005
Bruce B, Ph. BW. A Guide to Choosing a Particle Sizer
All Rights Reserved by Gold APP Instruments Corp. Ltd.
WeChat WhatsApp


GOLD APP INSTRUMENTS CORP. LTD.
HongKong Add: Flat Rm A17, Legend Tower, No. 7 Shing Yip Street, HK, China
Mainland Add: R1302, Baoli Tianyue, Shaowen Rd., Yanta Dist., Xi'an 710077, China
T: +86-182 0108 5158
E: sales@goldapp.com.cn